Throughout my research I kept finding recurring associations between Artemisia species and women’s health. All over the world, leaves have been used for a range of feminine needs such as abortions, treating menstrual cramps, reducing pain during childbirth, initiating birth, and postpartum care (I did not keep track of all the sources as I was not focusing on the wide array of Artemisia species, but evidence for this is easy to find). Riddle describes the namesake of Artemisia, “the Mother Herb”, as Greek goddess Artemis (2010). While popularly known as goddess of the hunt, Artemis was also responsible for women’s reproductive health (p. 80). Fittingly, the silvery leaves of many Artemisia species are associated with the moon, another symbol of Artemis. Multiple sources also describe Artemisia’s ability to ward off evil spirits and unwanted energies (Schofield, 2000, p.247; Wright, (2002).
In Alaska, Artemisia tilesii Ledeb. is the most salient Artemisia species. It is also the most abundant, ranging throughout all parts of Alaska. A. tilesii is the species growing along the road up to my house, at the edges of local beaches and the shale bluffs leading into the sea. Multiple sources describe Artemisia species as a self-limiting medicine; the bitterness of the tea discourages excessive consumption.
A. tilesii, often called stinkweed (or Sargiq in Inupiaq), is a medicine used for a wide variety of ailments by Inupiat people. Anore Jones explains that it is the first plant she uses for medicine when confronting an ailment (2009, p.160). The plant can be used externally as a rinse/poultice/wrap for skin problems or wounds, and internally for colds, coughs, etc. It has sometimes been used for more serious ailments such as cancer or blood poisoning. Jones recommends pouring boiling water over the leaves or boiling for five minutes, depending on preferred strength (2009, p.160) However, tea from the plant should only be drunk in small doses as a medicine, not for frequent use. The plant is harvested both fresh and dried. Dried leaves can be harvested year round when found poking out of the snow, since they remain on the stalks. Some elders believe that leaves dried on the plant lose some of the harmful volatile oils, but remain equally medicinal (p.160). I was curious to know more about this particular claim, but could find no information. When fresh leaves are used externally, they are crushed first.

Viereck describes that Dena’ina peoples have used A. tilesii in steam baths, the leaved stalk as a switch for arthritis, and the soaked leaves as a pain relief on pregnant bellies (1987, p.78).
Alutiiq peoples have also used the stalk as a switch for pain (Russel, 2017, pg.131). Tea from boiled leaves was used for a wide variety of ailments ranging from heart problems to hernias. People from Port Lions/Ouzinkie/central report rubbing dry leaves into a soft fuzz to apply to sores and cuts (p.132). Sometime people would nibble on dry or fresh leaves for pain relief.
Russel also notes that outer inlet Dena’ina people would wear frewsh leaves in their shoes to treat athlete’s foot and reduce smell (Kari, 1995, p.139). Inland Dena’ina would use crushed leaves to reduce itching and as a mosquito repellant.
Overfield et al. observed a Yupik girl using a pulp mixture of boiled A. tilesii leaves to heal a skin wound (1980, p.97). Flailing with stems after a sauna was also mentioned. A specific cold remedy was outlined: boil for 30 minutes, strain, take several teaspoons a day (p.98)
Schofield describes a variety of contemporary uses not particularly associated with cultural groups in Alaska. She suggests drinking 2 cups of A. tilesii tea (made with 1 tsp of dry herb per cup of water) to treat roundworms and pinworms (2000, p. 245). The tea is useful for stomach complaints and to regulate menstruation/reduce cramping. A steam with the plant can help relieve congestion (p.246). The powdered flowers can also be mixed in with dog food to treat worms. In the garden, chopped leaves can protect young cabbage plants from pests, or when mixed with sharp pebbles can deter slugs (p. 247).
Interestingly, alterations to the fresh leaf for external use is highly common. This makes me think that some chemical change occurs in the plant when crushed and soaked in water.

Physiology
Artemisia tilesii Ledeb. (Asteraceae) is a perennial herb that can grow stems horizontally above and below ground (Aiken et al.,1815). The root is somewhat woody with fibrous roots composing the primary root mass (although occasionally it can form a tap root) (Aiken et al.,1815). The plants are usually around 1 to 3 feet and like to grow in sandy soils, particularly near shorelines, disturbed areas, and alpine tundra (Jones, 2009; Shultz, 2006, p.533). Distal stems are sometimes haired, and the leaves are alternate on the stem (Shultz, 2006). Petioles, if present, are not winged (Aiken et al.,1815). The top of the leaf blade is green and smooth, while the undersides are matted with wooly white hairs (Shultz, 2006, p.533). Leaf variation for A. tilesii can be highly diverse, but they are generally pinnately lobed, irregular, and the blade edges curl slightly in towards the blade’s lower surface (Aiken et al.,1815). Upper leaves can be more linear (not lobed). Aiken et al. note that the blades are .75 inches to 3 in long, and .4 inches to 1.2 inches wide (1815). Artemisia tilesii flowers in mid summer to early fall, often in August, and (Shultz, 2006, p.533). Their pale yellow (or pink) composite flowers have small tubular disk florets forming the head (similar in appearance to the center of a daisy) (Aiken et al.,1815). I thought they looked like a cluster of semi-translucent larvae spilling from the bracts. The whorl of bracts (involucre) is bell shaped and the bracts are purple or brown and lightly haired (Shultz, 2006, p.533).


In personal observations, I’ve noticed that the uppermost leaves (those once wrapped around the flower heads before they develop) of the A. tilesii growing along my road have fine white hairs on the top of the blade as well as the bottom. I also observed many pre-flower plant tops enclosed in white fuzz. While I think the second observation is likely a result of insect or arachnid activity, I noticed the same white fuzz (appearing to be hairs) on upper leaves in pictures of A. tilesii.

Other Alaskan Artemisia species
When I began my research into the Artemisia species growing in Alaska, I only expected to be exploring several species. Immediately, the diversity of Artemisia here became clear. Using the Integrated Taxonomic Information System (ITIS), I composed a list of all accepted Artemisia species. I then submitted each species into the Global Biodiversity Information Facility (GBIF) occurrences map to determine its presence/absence and distribution in Alaska. I found there were 16 Artemisia species in Alaska, although some were only observed here a few times. I was surprised that many of the species seemed to have originated in Beringia (roughly the area composed by Kamchatka, Chukotka and Alaska). Riggins and Siegler found that the oldest Artemisia lineages can be found in Beringia (2012). The abundance of Artemisia pollen found in the area also serve as indicators of Alaska’s vastly different historical ecosystems. The strong ties between Artemisia species and Alaska made me feel a personal connection to the plant as I continued with my research. First I wanted to get a broad idea of what these different species looked like, to see how many of them were similar in appearance to Artemisia tilesii, which I am most familiar with.



My initial intention with the project was to compare the physiology and chemical composition of the predominant Alaskan wormwood (Artemisia tilessi L.) and an invasive (likely European) wormwood species. Using the GBIF database, I determined what species should be considered, and speculated on where species were initially from (using a timeline feature).
| Artemisia species
In Alaska |
Distribution | First Year Observed in AK | Likelihood of Growing in Homer (High, Medium, Low) |
| A. biennis Willd. | A handful of sightings in the western interior of AK, distributed more throughout Canada and mid-north US, and scattered in Europe and Asia, | Early 1950s. Possibly spread from Europe to the US to AK. | Low |
| A. vulgaris L. | Only 3 sightings in AK. One near Anchorage and two near Point Lay along the Northwestern coast. Otherwise distributed across US, heavily concentrated in the East, dense in Europe, distributed across Asia, and scattered in Northern South America, Sparse sightings in Canada. | Between 1680 and 1700. Possibly spread from Europe to the US to AK. | Very Low |
| A. alaskana Rydb. | Patchily dispersed across AK, in northwestern Canada, and Eastern Russia. | Early 1940s. First observations of species worldwide are in Alaska. | Low
(No sightings on the Kenai Peninsula) |
| A. tilesii Ledeb. | Heavily concentrated in Alaska, regular along circumpolar line, extending down through the Yukon and BC into the Washington state. | Late 1890s. Possibly spread from Russia, First observations are in Chukotka. | High (Sightings in Homer and the Kenai Peninsula) |
| A. aleutica Hultén | Only two sightings worldwide, both in Alaska, One is on Kiska island and one is on Hawadax island. Both islands are in the Aleutians and are very close together | 1985. First observations of species worldwide is in Alaska. | Very Low |
| A. borealis Pall. | Scattered throughout AK, eastern Russia, and Northern Canada. Rare in BC and US | Late 1920s. First sightings of species worldwide is in AK. | Low/Medium
(Two sightings in Anchorage) |
| A. dracunculus L | There are a handful of sightings in southern AK, and dispersed throughout southern Canada and the US. Dispersed across Asia and Russia, and denser in Europe. | Early 1960s.
Possibly spread from Europe through US to AK. |
Low |
| A. frigida Willd. | Moderate sightings in eastern AK, but sparse in the west. Dispersed throughout the Midwestern US, Canada, southern Russia, and northern Asia | Late 1920s.
First sightings worldwide are in the US. Possibly spread from US to AK. |
Medium
(Several sightings on Kenai Peninsula) |
| A. furcata M. Bieb. | Lightly dispersed through AK and in/around Chukotka. Sparse sightings in northern Canada and northern Russia, a handful in the northwest US. | Early 1940s. May have spread from either Russia or the US. | Medium/low
(Sightings around Anchorage) |
| A. globularia Cham. Ex Bess | Light dispersion through AK, mostly in the west and across Bering strait. | Late 1920s. First sighting worldwide are in Chukotka. Possibly spread from Russia. | Low |
| A. glomerata Ledeb. | Scattered in Chukotka, and northern AK. Sparse in Japan. | Late 1880s. First sighting worldwide is on the eastern edge of Chukotka. | Very Low |
| A. senjavinensis Besser | Sightings isolated to the Seward peninsula in AK and coastal Chukotka. | Late 1920s. First sighting worldwide is in Alaska. | Very Low |
| A. laciniata Willd. | Sparse in eastern interior of AK, dense in southern Russia, and rare in southwest US | Late 1950s. Possibly spread from Russia. | Low |
| A. michauxiana Besser | Dispersed along west coast of US and Canada. One sighting in AK. | 1989. Possibly spread from US. | Very low |
| A. norvegica R.E.Fr. | Regularly dispersed throughout Alaska, Chukotka and Western Canada, sparse in US | 1914. Observed first in mainland US and Eastern Chukotka. | Medium
(Sightings on Kenai Peninsula) |
| A. tridentata Nutt. | Dense in western half of US, two sightings near Utquiagvik | Early 1950s. First sightings worldwide were in US. | Very low |
Table 2. Artemisia species found in Alaska, and their distribution according to GBIF (2019). I have included speculations on species movements based on occurrence observations on GBIF over time. I have also estimated the likelihood of finding the species in Homer, Alaska based on the number of specimens noted in the Kenai Peninsula area.
Although both Artemisia biennis Willd. and Artemisia vulgaris L. are on the non-native plant species list (Alaska Center for Conservation Science, n.d.), neither felt appropriate for this study considering their minimal distribution in Alaska and low likelihood of encounter; in AK, there were 4 A. biennis specimens and 3 A. vulgaris specimens on GBIF (2019). I thought Artemisia campestris, native to Eurasia, might be a suitable candidate, until I discovered that the subspecies present in Alaska is not accepted, and is instead a synonym for Artemisia borealis (ITIS, n.d.). Considering that A. borealis was first identified in Alaska, it did not seem like a suitable species for comparison. No other species growing regularly in Alaska was non-native to North America or neighboring Russia. However, one of my primary questions was whether A. tilesii is an abortifacient like other European/Asian Artemisia species (such as A. vulgaris), so I focused my study in that direction.
Chemistry of Artemisia
After being sure the familiar local Artemisia species was A. tilesii, I went to harvest leaves with a couple of friends. I had found the spot when taking my dog for a walk down a long rural road that dissipated into a trail toward the beach. The bluff was eroding rapidly, and shelves of dirt and desperate tree roots had been carved into the hill. The area was south-facing, toward Kachemak bay, and you could be standing on duff or rocks or mud, all within 50 feet. Patches of wormwood followed the bluff, not too close to the edge, but not extending backwards either into the water-logged grasses and black spruce bog. I love the smell of dried wormwood, especially fresh off the stalk, so when we approached the first patch I crushed a handful of leaves in my hands and took a deep whiff. The smell left me surprised. There were strong notes of lemon in it that I did not remember ever smelling in A. tilesii leaves before. I made a friend smell the leaves, and she too identified a citrus smell she did not recognize. We had been to the same area maybe a month before, enjoying the aroma, and were quite baffled. We wondered: a different time of year? I sought a different patch to sample and found the lemony notes to be gone. We hopped from patch to patch, crumbling and sniffing different leaves. I could distinctly differentiate between plants with the lemon smell and the plants that smelled primarily of sage. We could not distinguish any correlating habitat, life stage, or size differences. Without testing the oil composition of plants along with accompanying sniff tests, there is no way to say if we were observing actual differences in plant terpene composition (terpenes are very smelly). I did think it was interesting, however, that plants so close together could smell so distinct.
I have never associated Artemisia species with sage, so at first I could not identify what the smell of A. tilesii reminded me of. In trying, I asked a friend what she thought and she suggested camphor, which is interesting, because several specimens of A. tilesii from the Yukon River delta contained trace amounts of camphor (Overfield et al., 1980). In assessing, I was getting whiffs of rosemary and when I looked up a general chemical composition of rosemary, I found that the major component in the oil of Salvia rosmarinus is cineole (also called eucalyptol), which was also found in the Yukon River delta specimens (Overfield et al., 1980; Jiang et al., 2011). (Sidenote: I had no idea that rosemary was in the Salvia genus, and it blew my mind). Realizing rosemary’s connection to sage plants, I eventually realized that the familiar smell I was noticing was that of sage (culinary and common desert sagebrush kind of smells). I should have realized sooner considering how many Artemisia species have “Sagebrush” in their name! In Salvia officinalis, the type of sage found in herb gardens, the major compounds found in the volatile oils are cineole, camphor, borneol, thujone, and isothujone, which are characteristic of many Artemisia species (Hamidpour et al., 2014; Cedarleaf et al., 1983). I’m so new to chemistry, that perhaps these chemical connections are not so remarkable, but they brought me moments of joy and excitement as other aspects of Artemisia chemistry overwhelmed me.
What I found while researching, is that is is extremely difficult (from my level of chemistry comprehension) to make any definitive statements about chemical comparisons in Artemisia species. Considering the dearth of information surrounding the components of A. tilesii, and the high variability in chemical composition within the same species from different environments, the more I learned, the more distant answers seemed.
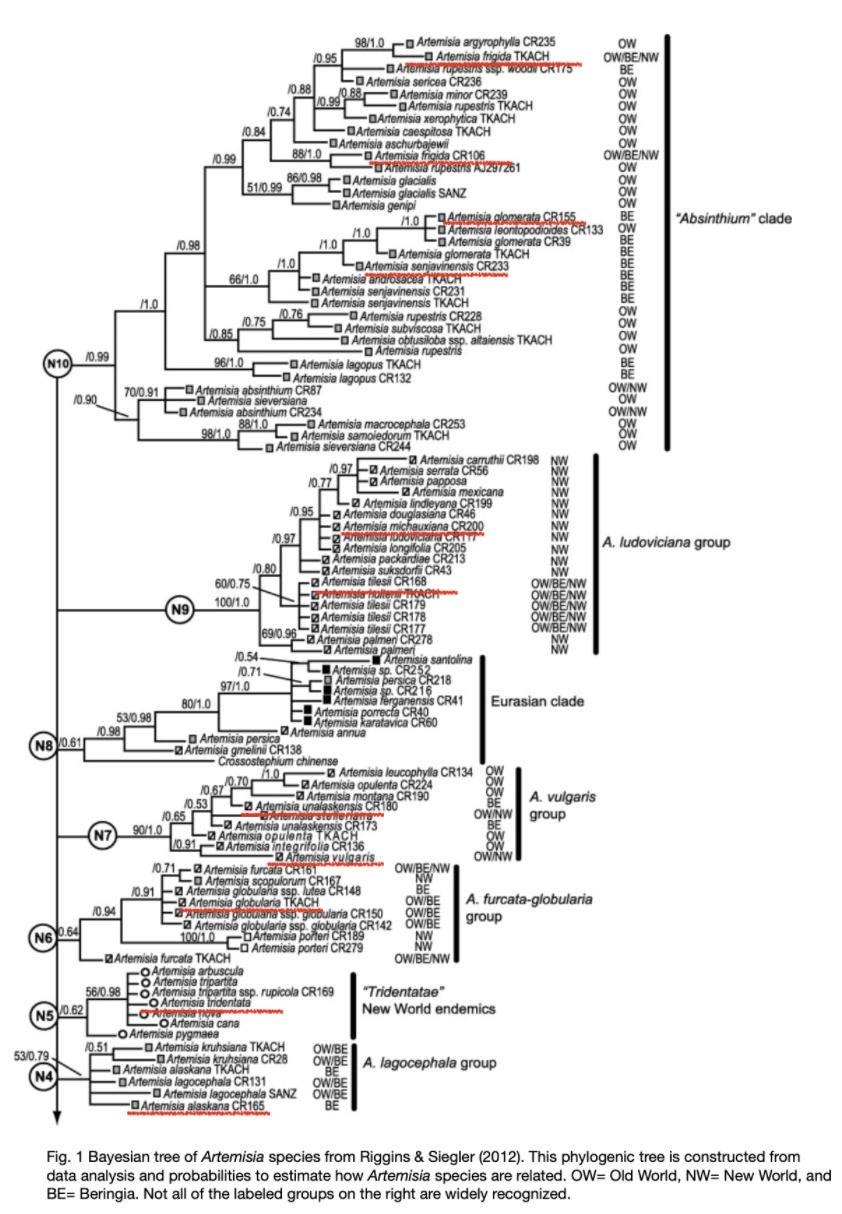
I thought understanding A. tilesii’s genetic connection to other species would help me determine its chemical components and their effects, but the chemical differences between species are too subtle to be characterized so broadly. However, the species classification is still interesting, and I have included a genetic tree (estimated) with the relevant Artemisia species underlined.
Artemisia species are often high in monoterpenes and sesquiterpenes, which are usually highly aromatic compounds. Overfield et al. tested the volatile oils of two Artemisia tilesii specimens from the Yukon River delta and found that thujone and isothujone were the primary constituents (1980, p.98). Cineole (also called eucalyptol), camphor, and artemisia ketone were found in trace amounts. Isothujone has pain-relieving properties similar to codeine, and thus is likely responsible for the effect on arthritis (Overfield et al., 1980, p.99). Overfield et al. also noted that the volatile portions of A. tilesii are nearly identical to Artemisia tridentata subsp. vasayana (Rydb.) Beetle (1980, p.98). Artemisia ketone demonstrates strong antimicrobial properties and can help protect cells from unstable, cancerous molecules (Lutgen, 2013).
Overfield et al. suggest that all of the volatile compounds they identified would only be present in preparations without heat, such as switching (1980, p.99). This makes me want to clarify that their expectation is correct and to explore what medicinal compounds might be acting on individuals after boiling. Heat would definitively alter the compounds available in the plant, so boiling A. tilesii leaves or simply soaking them in water before use would have different effects.
In further explorations, I would like to look into how various methods of preparing wormwood medicine impacts the active chemicals, and try to determine what compounds might be actively benefitting those who use the medicine.
Is Artemisia tilesii an Abortifacient?
No texts discussing use of Artemisia tilesii warn of abortifacient properties. However, the widespread use of many Artemisia species for abortion warrants the question. Trendafilova et al. suggest that a high camphor content in Artemisia species is responsible for its abortifacient properties (2021). Considering the strong correlation between the volatile components of A. tridentata and A. tilesii, I tried researching whether A. tridentata was ever used for abortions, but found no indication that it had. One study found that A. tridentata consumption did not cause abortions in sheep, (which are interestingly used to study human pregnancy), but was fatal in large doses when not eaten in smaller doses prior (Johnson et al., 1975). Volatile compounds are only one aspect of a plant’s properties, however, and it is possible that a different type of compound is responsible for abortifacient effects. Artimisinin, a sesquiterpene lactone found in some Artemisia species can be an abortifacient, especially in the early stages of pregnancy (Koe, 2020). It seems unlikely that A. tilesii contains artimisinin, considering its absence in a study on the sesquiterpene lactones in A. tilesii (Herz & Ueda, 1960). Interestingly, Artemisia species high in artemisinin usually do not contain artemisia ketone (Lutgen, 2013), a compound found in A. tilesii. The sesquiterpene lactone identified in Herz and Ueda’s paper was artilesin, also called matricarin (p.1140), so I looked for any correlations with that compound and miscarriage, but found none (although information was sparse). Although the trace camphor found in Artemisia tilesii by Overfield et al. is likely insufficient to cause an abortion, the variability in volatile compounds in other Artemisia species warrants caution. Further, the high variability of compounds in different Artemisia species suggests the possibility of several different compounds responsible for miscarriage.
There is strong evidence for chemical variability between Artemisia species growing in different environments, and over the lifecycle of the plant. Younsi et al. demonstrated that some environmental factors, such as rainfall and altitude, can influence the compounds present in essential oils in Artemisia campestris and Artemisia herba-alba (2017). Chemical compositions of A. campestris leaves in Libya and in Montenegro had different chemical compositions; while both had alpha and beta pinene, only one had isothujone, only one had camphor, and there were many other dissimilarities (2019). With such variability in mind, it is important to consider the correlation between traditional plant uses and their particular ecosystems.
A study by Cedarleaf et al. noted that A. tridentata had its highest monoterpine content in July, and its lowest in may (1983). Wright found there was higher thujone content in immature A. vulgaris plants (2002). Thao et al. found that the monoterpene content of the aerial parts of A. vulgaris changed over the course of seasonal growth, although the plant’s major characteristic compounds, (in this case camphor and eucalyptol), remained dominant (2004). If A. tilesii changes similarly, I could infer that thujone and isothujone would remain dominant compounds over the plant’s lifecycle, but that camphor, eucalyptol, and artemisia ketone percentages would vary.
Based on what I have learned, I would not trust Artemisia tilesii to act as an effective abortifacient. However, environmental impact on chemical variability and the absence of thorough investigations into A. tilesii chemistry are both sufficient cause to avoid internal use of A. tilesii medicines while pregnant.

Citations:
Aiken, S.G., Dallwitz, M.J., Consaul, L.L., McJannet, C.L, Boles, R.L., Argus G.W., Gillett, J.M., Scott, P.J., Elven, R., LeBlanc, M.C., Gillespie, L.J., Brysting, A.K., Solstad, H., & Harris, J.G. (1815). Flora of the Canadian arctic archipelago. NRC Research Press, National Research Council of Canada, Ottawa. https://nature.ca/aaflora/data/www/asatti.htm
Alaska Center for Conservation Science. Non-native plant species list. University of Alaska Anchorage. https://accs.uaa.alaska.edu/invasive-species/non-native-plant-species-list/
Cedarleaf, J. D., Welch, B. L., & Brotherson, J. D. (1983). Seasonal variation of monoterpenoids in big sagebrush [Artemisia tridentata]. Journal of Range Management, 36(4), 492-494. DOI: 10.2307/3897950
Global Biodiversity Information Facility. (2019). Occurrences. GBIF. https://www.gbif.org/occurrence/map?occurrence_status=present
Hamidpour, M., Hamidpour, R., Hamidpour, S. & Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. Journal of Traditional and Complementary Medicine, 4(2), 82-88. https://doi.org/10.4103/2225-4110.130373
Herz, W. & Ueda, K. (1961).The sesquiterpene lactones of Artemisia tilesii Ledeb. Journal of the American Chemical Society, 83(5), 1139-1143. DOI: 10.1021/ja01466a032
Integrated Taxonomic Information System. Home. ITIS. https://www.itis.gov/
Janaćkovića, P. , Rajčevića, N., Gavrilovića, M., Novakovića, J., Giwelib, A., Steševićc, D. & Marin, P.D. (2019). Essential oil composition of five Artemisia (Compositae) species in regards to chemophenetics. Biochemical Systematics and Ecology, 87. DOI: 10.1016/j.bse.2019.103960
Jiang, Y., Wu, N., Fu, Y., Wang, W., Luo, M., Zhao, C., Zu, Y., & Liu, X. (2011). Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environmental Toxicology and Pharmacology, 32(1). doi: 10.1016/j.etap.2011.03.011.
Johnson, A.E., James, L.F., & Spillett, J. (1975). The abortifacient and toxic effects of big sagebrush (Artemisia tridentata) and juniper (Juniperus osteosperma) on domestic sheep. Journal of Range Management, 29(4), 278-280. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C48&q=The+Abortifacient+and+Toxic+Effects+of+Big+Sagebrush+%28Artemisia+tridentata%29+and+Juniper%28Juniperus+osteosperma%29+on+Domestic+Sheep&btnG=
Jones, A. (2010). Plants that we eat. University of Alaska Press.
Kari, P.R. (1995). TANAINA PLANTLORE: Dena’ina k’et’una. Adult Literacy Laboratory, University of Alaska, Anchorage.
Koe, T. (2020, October 29). Miscarriage risk: TGA to investigate artemisia species permitted for use in supplements. Nutra ingredients. https://www.nutraingredients-asia.com/Article/2020/10/29/Miscarriage-risk-TGA-to-investigate-artemisia-species-permitted-for-use-in-supplements#:~:text=One%20of%20the%20chemicals%20found,first%20three%20months%20of%20pregnancy.
Lutgen, P. (2013, September 11). Artemisia ketone, phytosterols and lipid metabolism. Malaria World. https://malariaworld.org/blog/artemisia-ketone-phytosterols-and-lipid-metabolism
Overfield, T., Epstein, W.W., & Gaudioso, L.A. (1980). Eskimo uses of Artemisia tilesii (Compositae). Economic Botany, 34(2), 97-100. Stable URL: https://www.jstor.org/stable/4254154
Riddle, J.M. (2010). Artemisia, the “Mother Herb”. In: Goddesses, Elixirs, and Witches. Palgrave Macmillan. https://doi.org/10.1057/9780230105515_5
Riggins, C.W., & Siegler, D.S. (2012). The genus Artemisia (Asteraceae: Anthemideae) at a continental crossroads: Molecular insights into migrations, disjunctions, and reticulations among Old and New World species from a Beringian perspective. Molecular phylogenetics and evolution, 64(3). https://doi.org/10.1016/j.ympev.2012.05.003
Russel, P. (2017). Naut’staarpet- Our plants: A Kodiak Alutiiq plantlore. Alutiiq Museum & Archaeological Repository.
Schofield, J. (1989). Discovering wild plants. Alaska Northwest Books.
Shultz, L.M. Artemisia. US Fish and Wildlife Service. https://www.fws.gov/southwest/es/documents/R2ES/LitCited/LPC_2012/Shultz_2006.pdf
Thao, N.T.P., Thuy, N.T., Hoi, T.M., & Thai, T.H. (2004). Artemisia vulgaris L. from Vietnam: Chemical variability and composition of the oil along the vegetative life of the plant. Journal of Essential Oil Research, 16(4), 358-361. https://doi.org/10.1080/10412905.2004.9698742
Trendafilova, A., Moujir, L.M., Sousa, P.M.C. & Seca, A.M.L. (2021). Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods, 10(1), 65. https://doi.org/10.3390/foods10010065
Viereck, E.G. (1987). Alaska’s wilderness medicines: Healthful plants of the far north. Alaska Northwest Books.
Wright, C. (2002). Artemisia. Taylor & Francis Inc. https://books.google.com/books?hl=en&lr=&id=pZoz0VXr15wC&oi=fnd&pg=PP1&dq=Artemisia&ots=W3dWdg_cqO&sig=yLOMkM7G5VnNPVmc4aVqBIzdmnA#v=onepage&q=Artemisia&f=false
Younsi, F., Mehdi, S., Aissi, O., Rahali, N., Jaouadi, R., Boussaid, M., & Messaoud, C. (2017). Essential Oil Variability in Natural Populations of Artemisia campestris (L.) and Artemisia herba‐alba (Asso) and Incidence on Antiacetylcholinesterase and Antioxidant Activities. Chemistry & Biodiversity, 14(7). https://doi-org.prxy.lib.unbc.ca/10.1002/cbdv.201700017
Photograph Citations:
Bent, Julia. (2013). Artemisia furcata [Photograph]. Burke Herbarium Image Collection. http://biology.burke.washington.edu/herbarium/imagecollection/photo.php?Photo=wtu044252&Taxon=Artemisia%20furcata&SourcePage=taxon
Carlson,M., Lipkin, R., Cortes-Burns, H., & Lapina, I.V. (2006). Artemisia senjavensis [Photograph]. Stewart River Training Area Rare Plant Survey. https://accs.uaa.alaska.edu/wp-content/uploads/Stewart_River_Training_Area_Rare_Plant_Survey.pdf
Carr, G. (2021). Leaves: Artemisia tridentata [Photograph]. Go Botany. https://gobotany.nativeplanttrust.org/species/artemisia/tridentata/
Carr, R.L. (2011). Artemisia michauxiana [Photograph]. Burke Herbarium Image Collection. https://biology.burke.washington.edu/herbarium/imagecollection/photo.php?Photo=wtu050233&Taxon=Artemisia%20michauxiana&SourcePage=photos
Cook, A. (2009). Artemisia tilesii [Photograph]. Alaska wildflowers. http://www.alaskawildflowers.us/Kingdom/Plantae/Magnoliophyta/Magnoliopsida/Asteraceae/Artemisia_tilesii/Tilesii_14.html
Denver Botanic Gardens. (N.d.). Artemisia glomerata [Photograph]. Gardens Navigator. http://navigate.botanicgardens.org/weboi/oecgi2.exe/INET_ECM_DispPl?NAMENUM=16615
Hogan, T. (2010). Artemisia laciniata subsp. Parryi [Photograph]. University of Colorado Museum of Natural History Herbarium Vascular Plant Collection. https://intermountainbiota.org/portal/collections/individual/index.php?occid=13870302
Harte, M.E. (N.d.). Aleutian wormwood [Photograph]. Bugwood. https://www.forestryimages.org/browse/detail.cfm?imgnum=5425196
Harte, M.E. (2021). Stems: Artemisia frigida [Photograph]. Bugwood. https://gobotany.nativeplanttrust.org/species/artemisia/frigida/
Lovit, M. (2021). Leaves: Artemisia vulgaris [Photograph]. Go Botany. https://gobotany.nativeplanttrust.org/species/artemisia/vulgaris/
Mittelhauser, G. (2021). Leaves: Artemisia biennis [Photograph]. Go Botany. https://gobotany.nativeplanttrust.org/species/artemisia/biennis/
Morse, K. (2021). Leaves: Artemisia dracunculus [Photograph]. Go Botany. https://gobotany.nativeplanttrust.org/species/artemisia/dracunculus/
Northland Arts Nature Images. (2014). Artemisia globularia [Photograph]. Flora, Shrubs & Trees of Alaska. https://www.northlandartsnatureimages.com/Nature/Flora-Fungii/i-sttxKkQ
Southwest Colorado Wildflowers. (2012). Artemisia borealis [Photograph]. SW Colorado Wildflowers. https://www.swcoloradowildflowers.com/Yellow%20Enlarged%20Photo%20Pages/artemisia%20borealis.htm
Tracy, D. (2016). Artemisia norvegica [Photograph]. Burke Herbarium Image Collection. http://biology.burke.washington.edu/herbarium/imagecollection/photo.php?Photo=wtu056231&Taxon=Artemisia%20norvegica&SourcePage=taxon
Viereck, E.G. (1987). Alaska’s wilderness medicines: Healthful plants of the far north. Alaska Northwest Books.
Posted: February 2021.